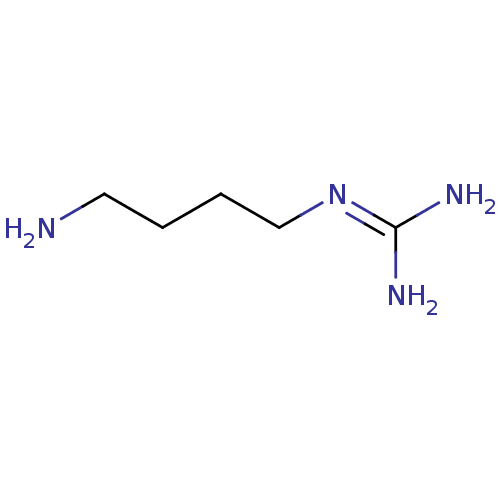
BDBM85213 Agmatine::CAS_306-60-5::CHEMBL58343::NSC_199::US8633208, Agmatine
SMILES [#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7]
InChI Key InChIKey=XWVGKWZOVAVNBI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 85213
Found 11 hits for monomerid = 85213
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity of compounds was evaluated by a homogeneous luminescent method, the MAO-Glo Assay (Promega), measuring the monoamine oxidase acti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity of compounds was evaluated by a homogeneous luminescent method, the MAO-Glo Assay (Promega), measuring the monoamine oxidase acti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+5nMAssay Description:In vitro IC50 value by measuring the inhibition of deoxyhypusine synthase.More data for this Ligand-Target Pair
Affinity DataIC50: 3.65E+4nMAssay Description:Displacement of [3H]clonidine from imidazoline I1 receptor in Wistar rat kidney by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.65E+4nMAssay Description:Inhibition of Wistar rat imidazoline I1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMpH: 6.5Assay Description:Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot methodMore data for this Ligand-Target Pair
TargetIonotropic glutamate receptor subunit Delta2(African clawed frog)
State University of New York
Curated by PDSP Ki Database
State University of New York
Curated by PDSP Ki Database
Affinity DataKi: 8.60E+6nMAssay Description:TP_TRANSPORTER: inhibition of uptake of 0.1 uM MPP+ in OCT1-expressing 293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+7nMAssay Description:TP_TRANSPORTER: inhibition of uptake of 0.1 uM MPP+ in OCT1-expressing 293 cellsMore data for this Ligand-Target Pair