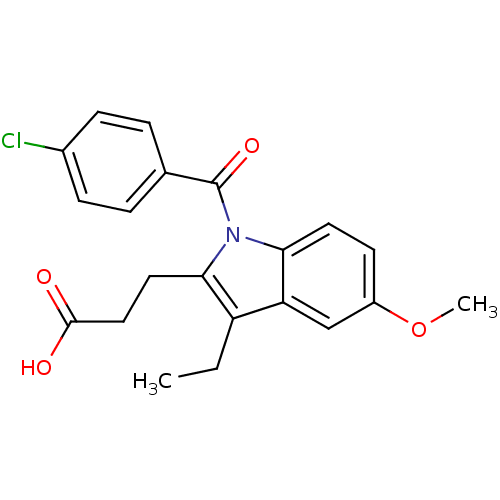
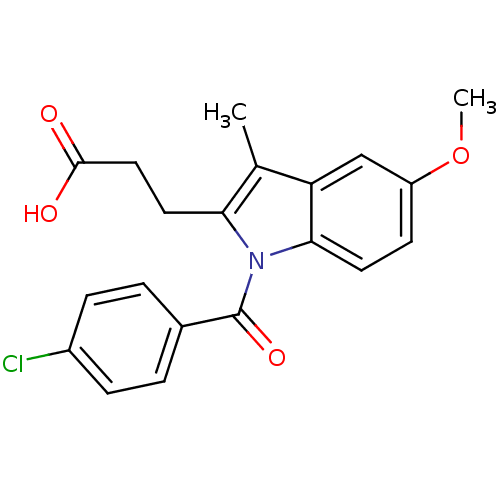
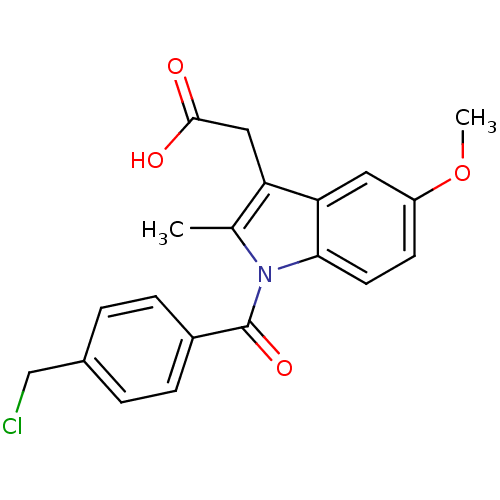
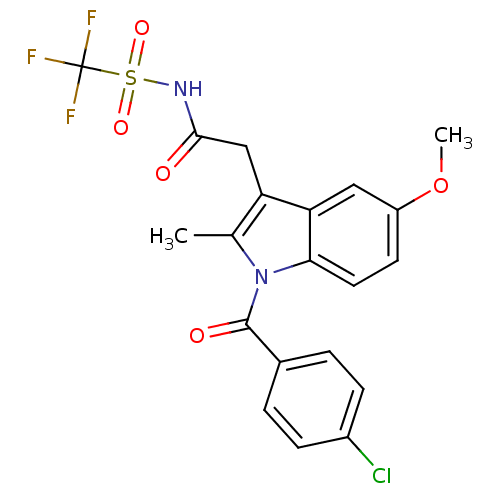
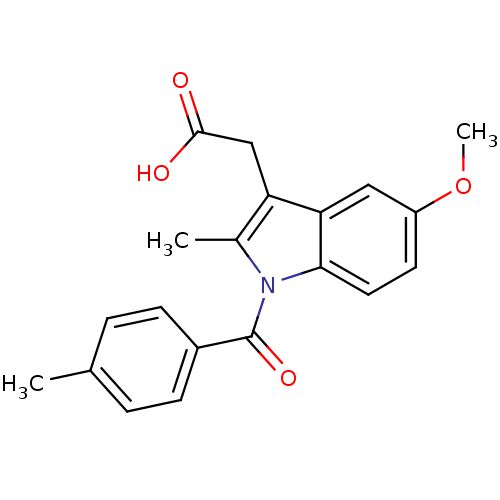
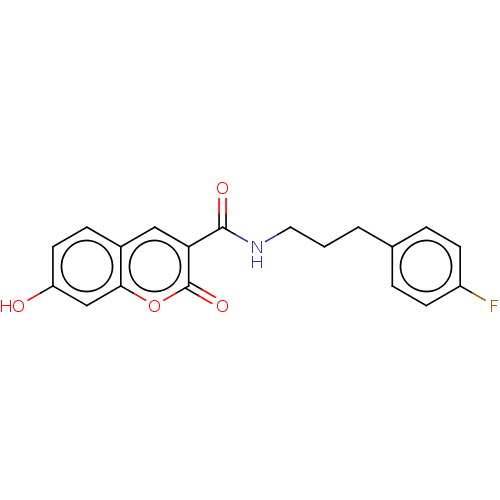
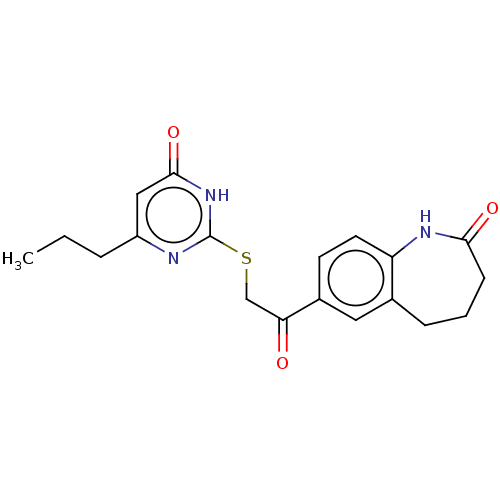
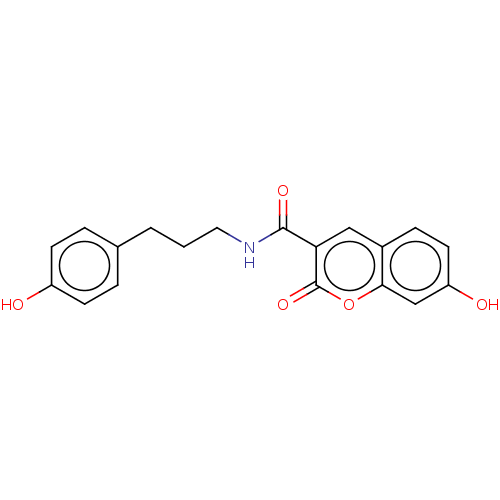
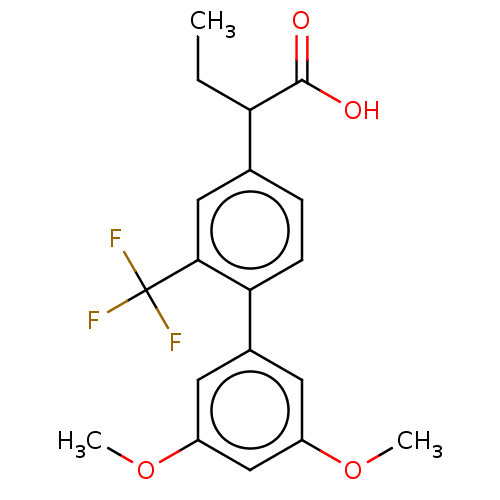
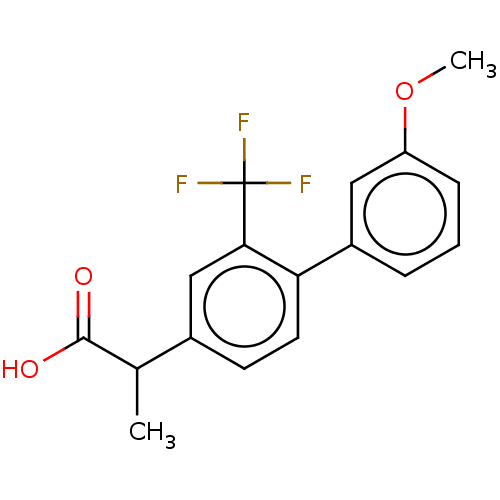
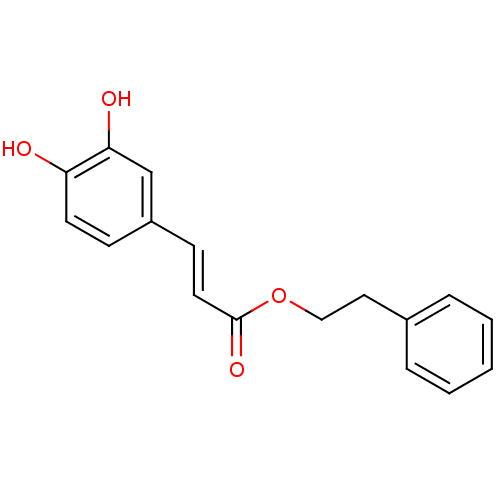
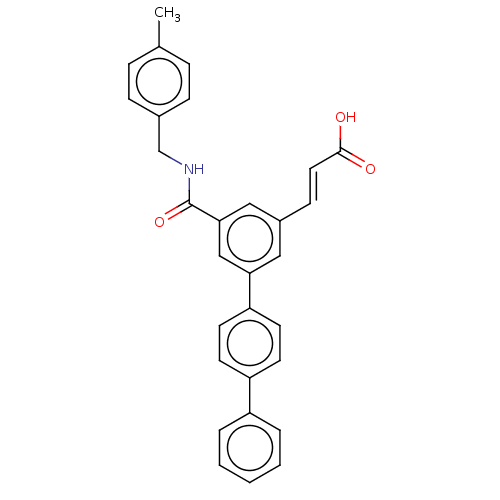
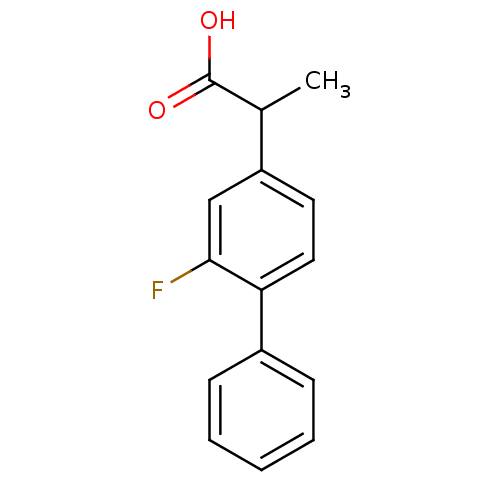
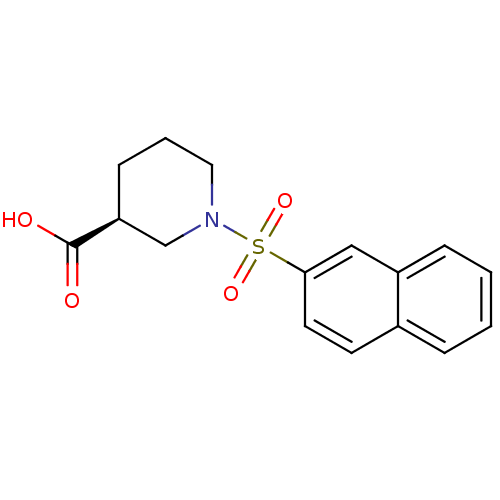
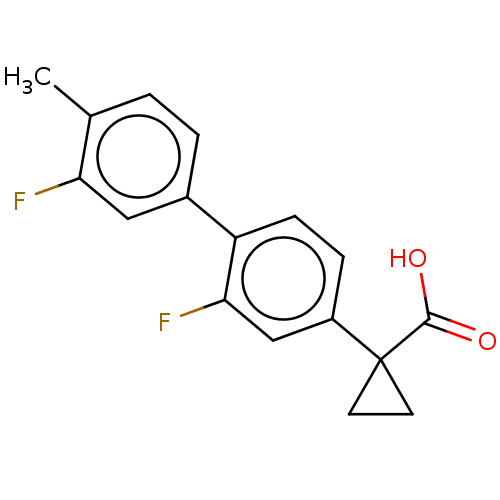
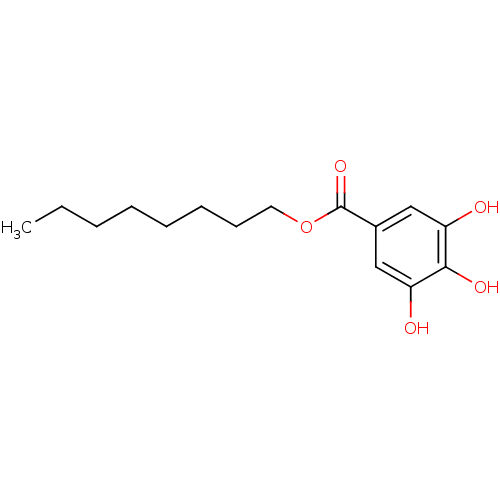
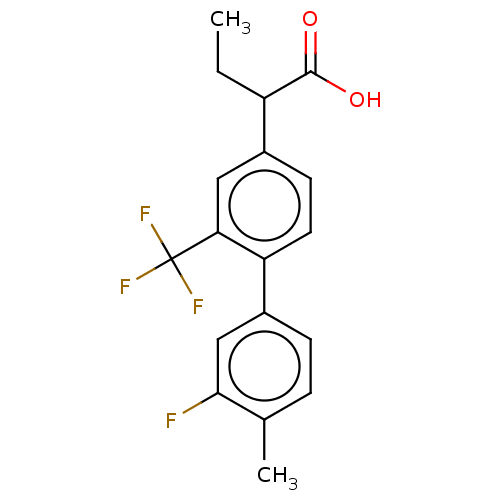
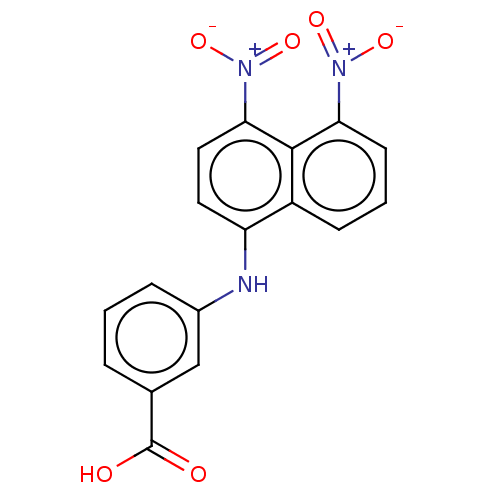
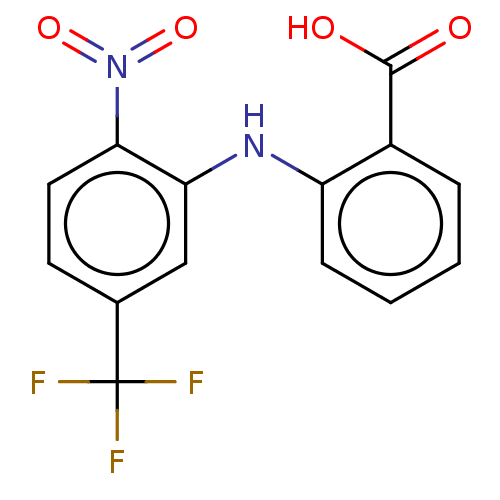
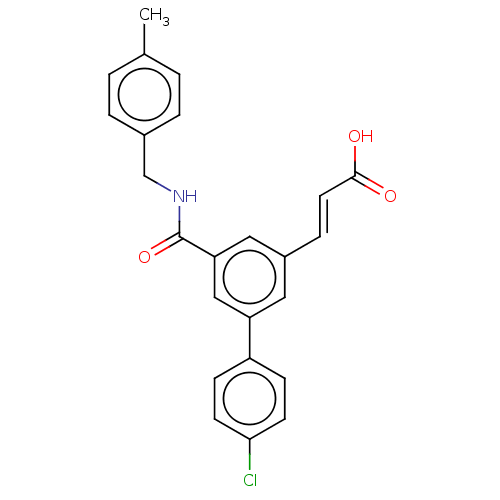
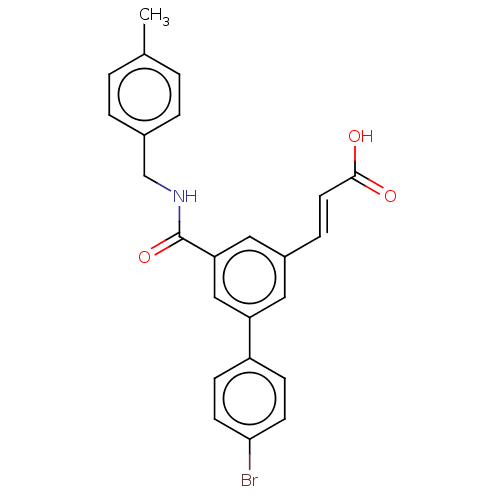
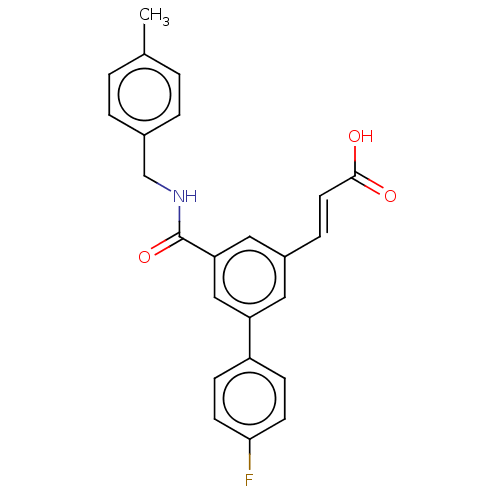
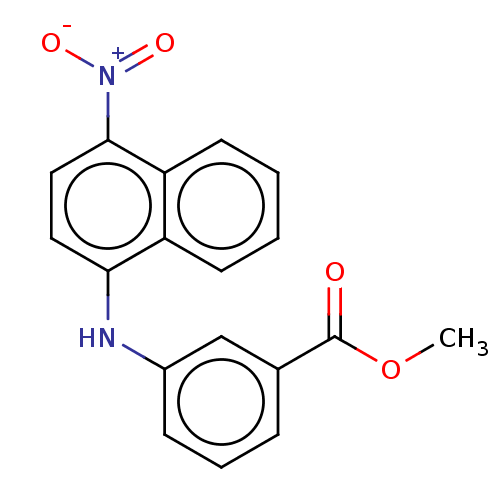
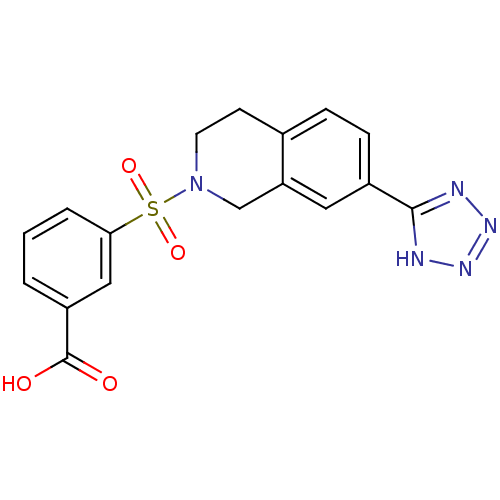
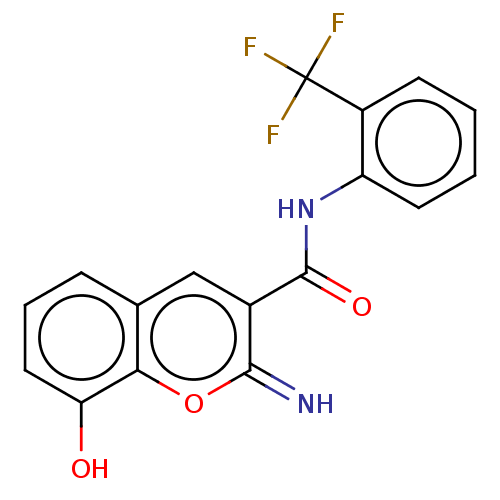
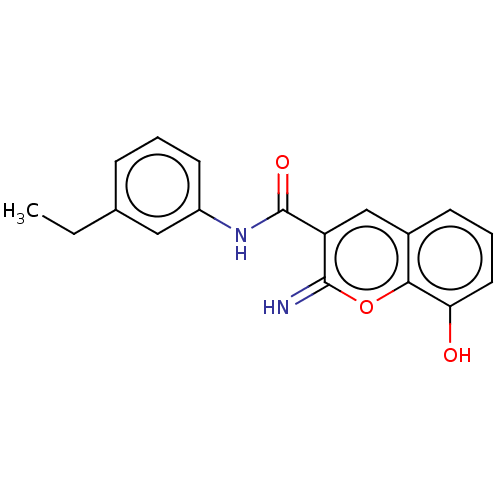
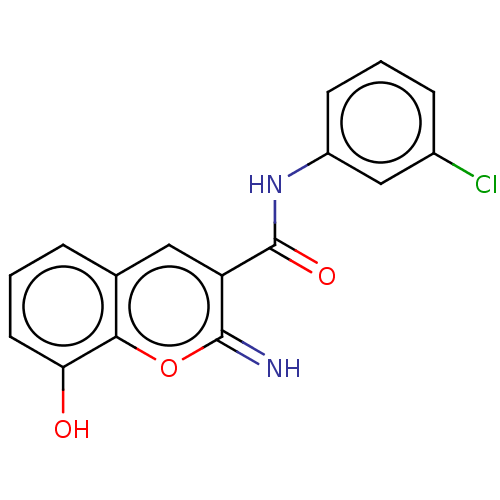
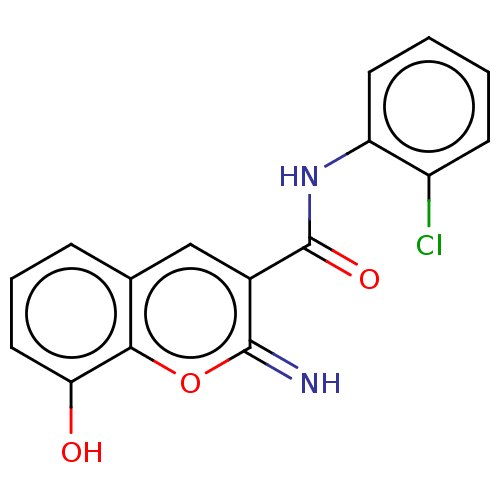
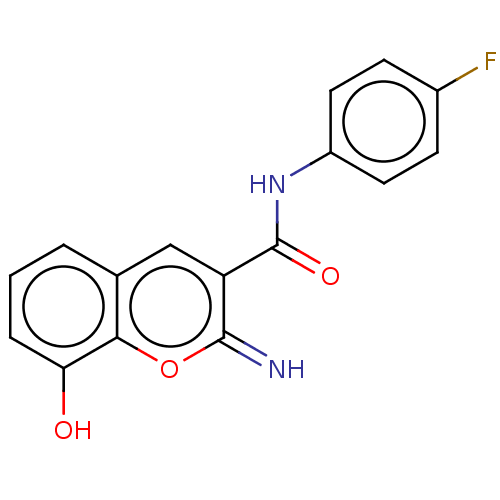
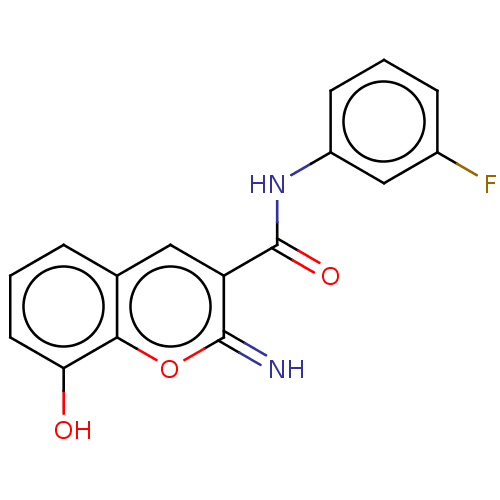
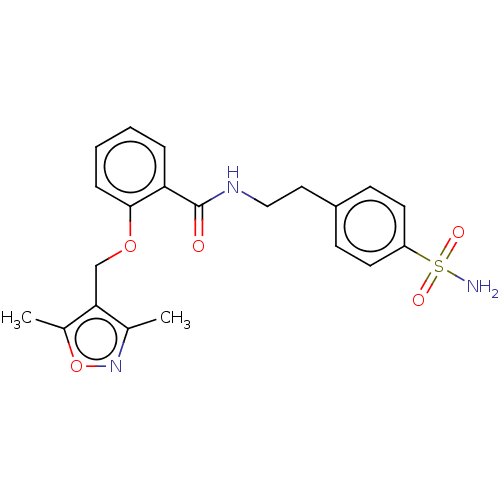
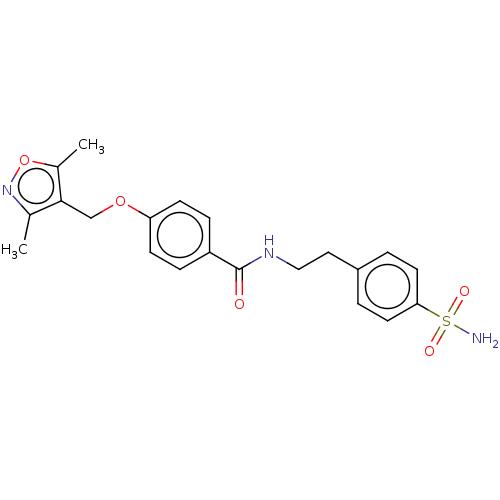
Affinity DataIC50: 1.95nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.15nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.51nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 12.6nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 48.7nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 49.8nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of recombinant human AKR1C4 using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells assessed as inhibition of NADP+ dependent oxi...More data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells assessed as inhibition of NADP+ dependent oxi...More data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Inhibition of recombinant human AKR1C4 using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.24E+3nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells using S-tetralol as substrate assessed as inh...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.27E+3nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells using S-tetralol as substrate assessed as inh...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.56E+3nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells using S-tetralol as substrate assessed as inh...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.95E+3nMAssay Description:Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human recombinant GST-tagged AKR1C4 expressed in Escherichia coli using S-tetralol as substrate by fluorometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) dehydrogenase activity by measuring NADH formation by spectrophotometryMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 2.98E+3nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells using S-tetralol as substrate assessed as inh...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
Affinity DataIC50: 3.15E+3nMAssay Description:Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: 3.51E+3nMAssay Description:Inhibition of human recombinant AKR1C4-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: 3.95E+3nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells using S-tetralol as substrate assessed as inh...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 4.04E+3nMAssay Description:Inhibition of N-terminal His-tagged human AKR1C4 expressed in Escherichia coli BL21 (Condon Plus) competent cells using 9,10 -Phenanthrenequinone as ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.67E+3nMAssay Description:Inhibition of human recombinant AKR1C4 transfected in Escherichia coli BL21 (DE3) pLysS competent cells using S-tetralol as substrate assessed as inh...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 5.34E+3nMAssay Description:Inhibition of human AKR1C4 using S-tetralol as substrate in presence of NADP by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) dehydrogenase activity by measuring NADH formation by spectrophotometryMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) dehydrogenase activity by measuring NADH formation by spectrophotometryMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) dehydrogenase activity by measuring NADH formation by spectrophotometryMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) dehydrogenase activity by measuring NADH formation by spectrophotometryMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 8.17E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+3nMAssay Description:Inhibition of recombinant AKR1C4 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.25E+3nMAssay Description:Inhibition of N-terminal His-tagged human AKR1C4 expressed in Escherichia coli BL21 (Condon Plus) competent cells using 9,10 -Phenanthrenequinone as ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.81E+3nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C4 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant AKR1C4 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH levelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AKR1C4 (unknown origin) incubated for 10 mins by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AKR1C4 (unknown origin) incubated for 10 mins by fluorometric analysisMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AKR1C4 (unknown origin) incubated for 10 mins by fluorometric analysisMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AKR1C4 (unknown origin) incubated for 10 mins by fluorometric analysisMore data for this Ligand-Target Pair