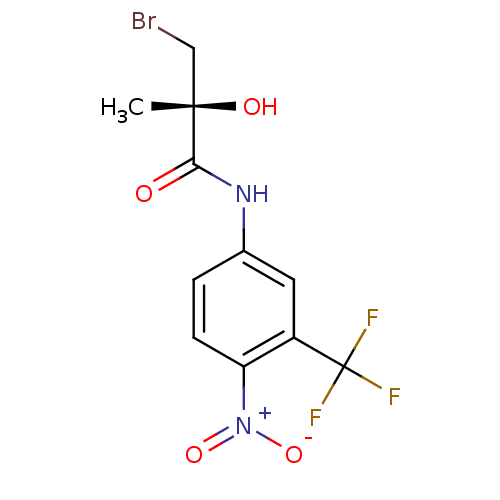
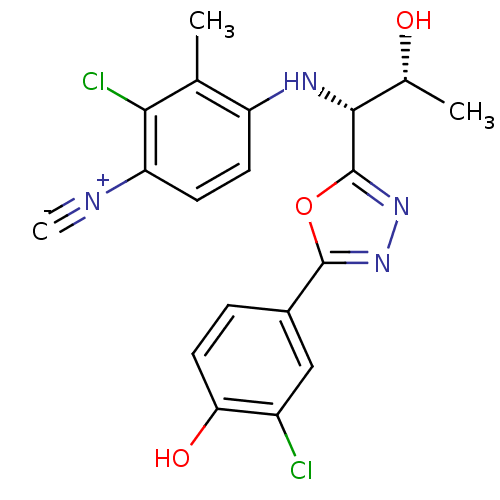
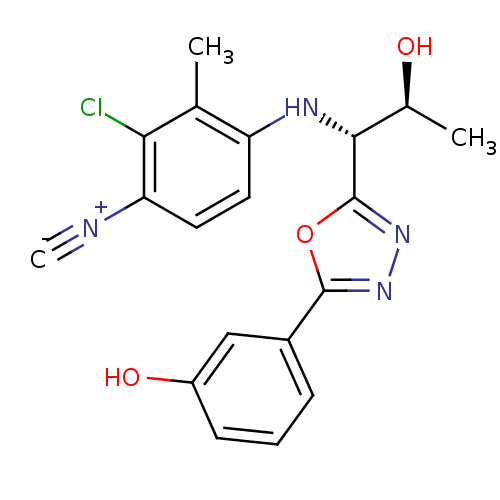
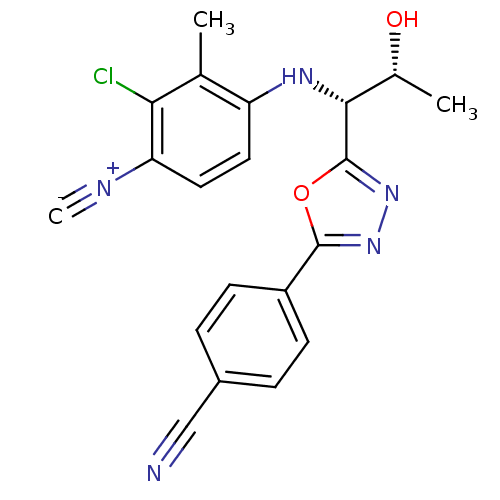
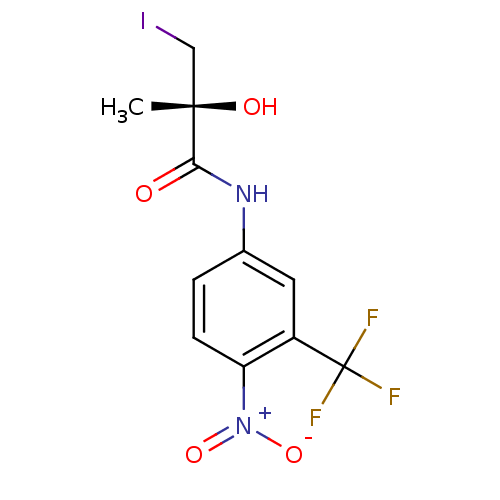
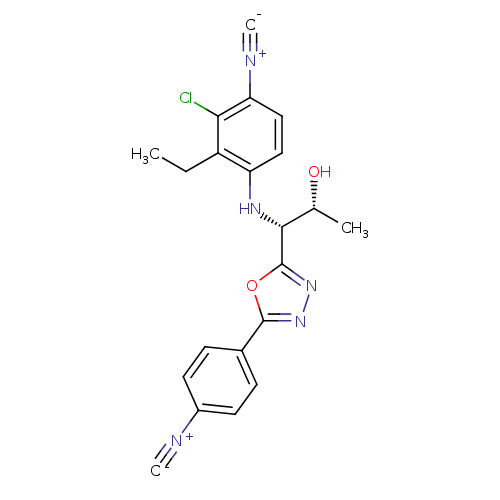
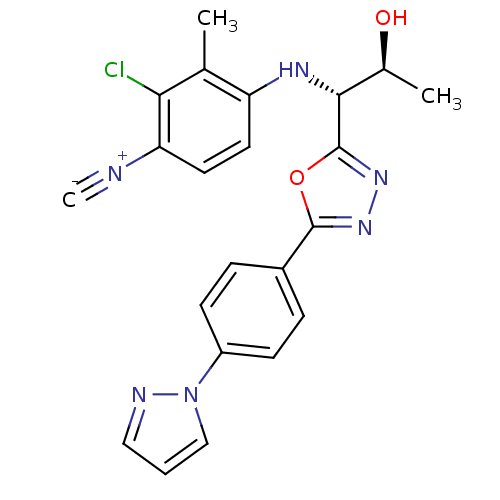
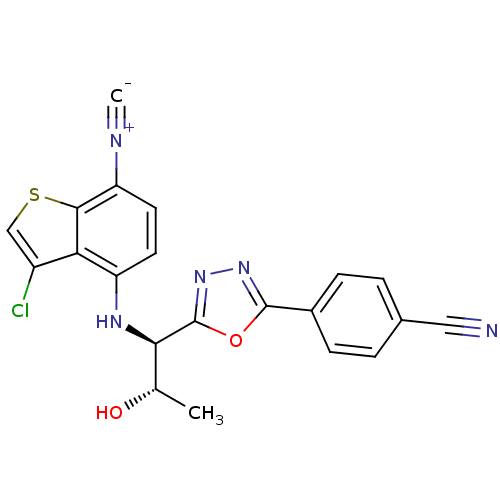
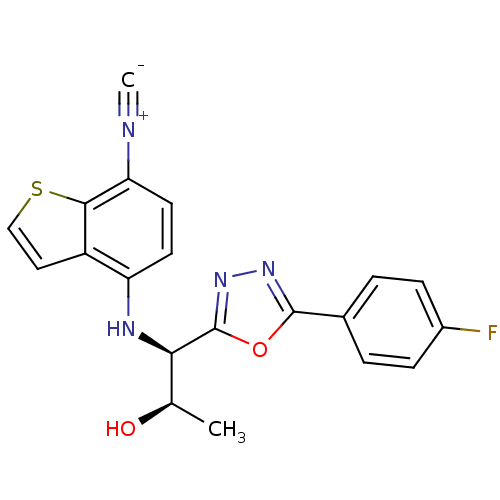
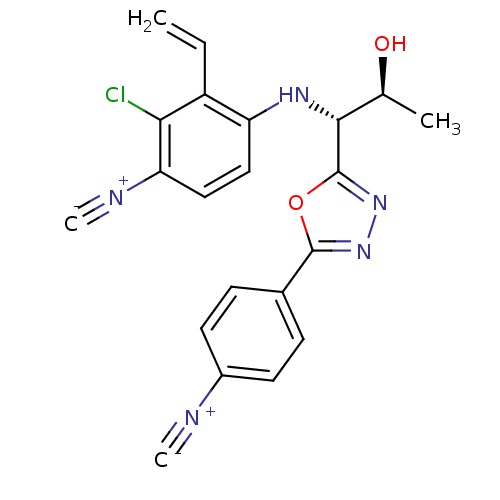
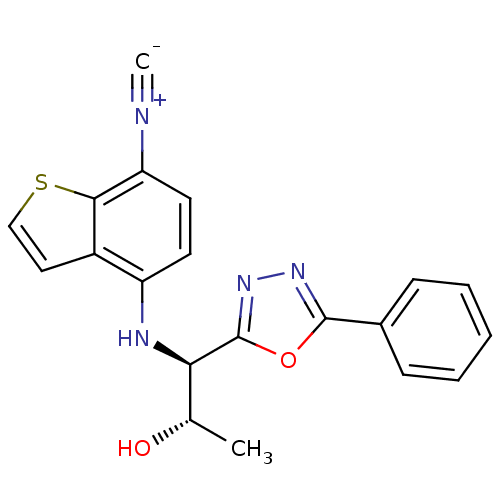
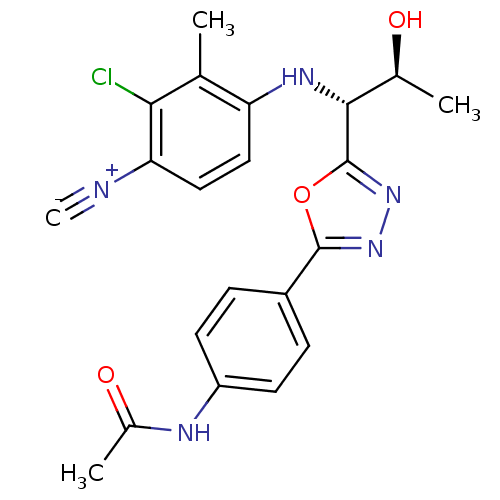
Report error Found 847 of affinity data for UniProtKB/TrEMBL: P15207
Displayed 1 to 50 (of 847 total ) | Next | Last >>

Affinity DataIC50: 0.0200nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataKi: 0.270nM ΔG°: -50.8kJ/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 0.280nM ΔG°: -50.7kJ/mole EC50: 1nMpH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 0.282nMAssay Description:Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostateMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]-MIB from cytosolic Androgen receptor derived from Sprague-Dawley rat prostate in presence of triamcinolone by competitive bindin...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -50.5kJ/mole EC50: 500nMpH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataKi: 0.302nMAssay Description:Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataKi: 0.430nM ΔG°: -49.7kJ/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataKi: 0.540nM ΔG°: -49.2kJ/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -48.9kJ/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -48.9kJ/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:Inhibitory constant against rat prostate cytosol androgen receptor using [3H]miboleroneMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataKi: 0.708nMAssay Description:Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostateMore data for this Ligand-Target Pair
Affinity DataKi: 0.710nMAssay Description:Inhibition of rat prostate cytosolic androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.710nMAssay Description:Inhibitory constant against rat prostate cytosol androgen receptor using [3H]miboleroneMore data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Inhibition of rat prostate cytosolic androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Inhibitory constant against rat prostate cytosol androgen receptor using [3H]miboleroneMore data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Binding affinity against rat prostate cytosolic Androgen receptor using [3H]mibolerone as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.759nMAssay Description:Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostateMore data for this Ligand-Target Pair
Affinity DataKi: 0.851nMAssay Description:Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostateMore data for this Ligand-Target Pair
Affinity DataKi: 0.860nM ΔG°: -48.1kJ/mole EC50: 500nMpH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataEC50: 0.970nMAssay Description:Agonist activity at rat androgen receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]-MIB from wild-type rat AR LBD measured after 16 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]-MIB from rat prostate cytosolic androgen receptor by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -47.0kJ/molepH: 7.4 T: 2°CAssay Description:The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibitory constant against rat prostate cytosol androgen receptor using [3H]miboleroneMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of [3H]mibolerone binding to cytosolic androgen receptor of rat ventral prostateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Binding affinity to rat Androgen receptor in African green monkey COS-7 cells measured by whole-cell binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of rat AR-mediated reporter gene expression in COS7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:In vitro antagonist activity against rat prostatic androgen receptor (AR)More data for this Ligand-Target Pair
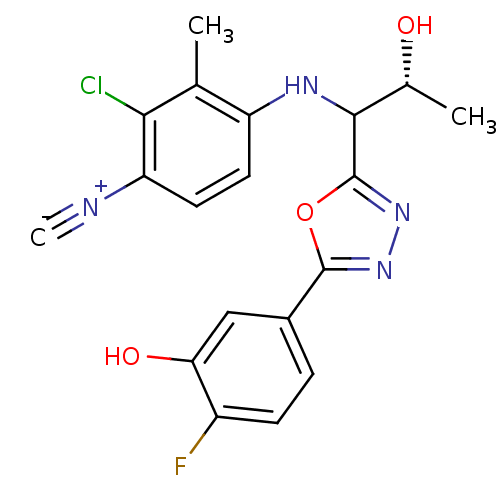
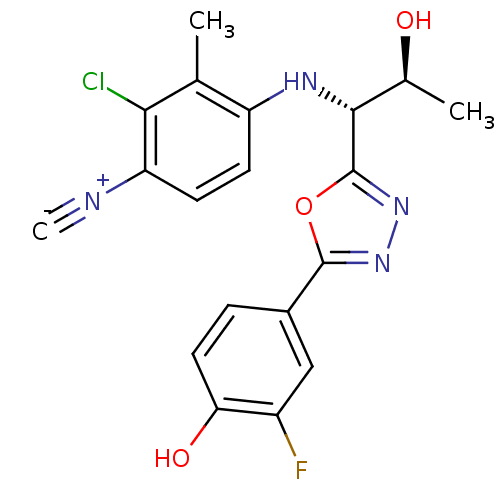
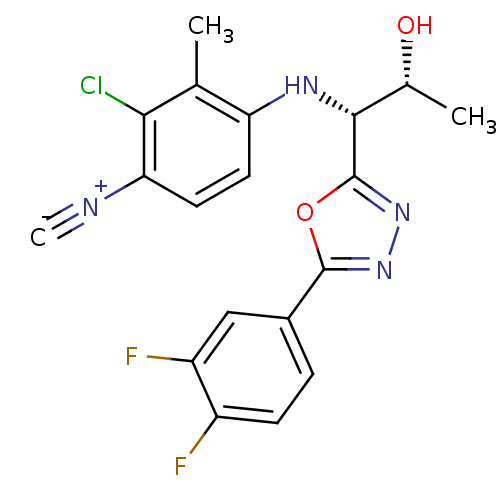
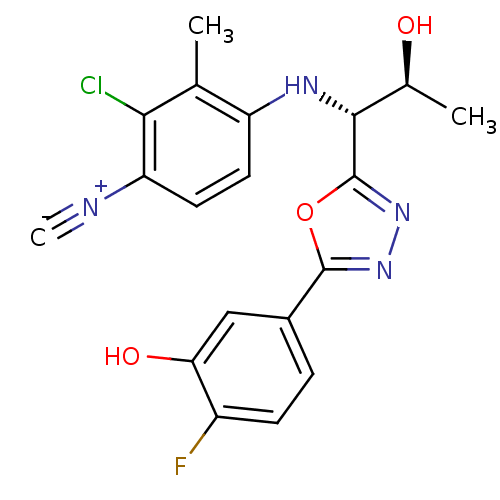
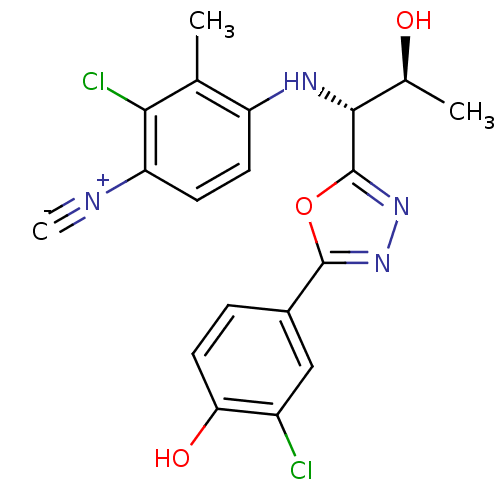
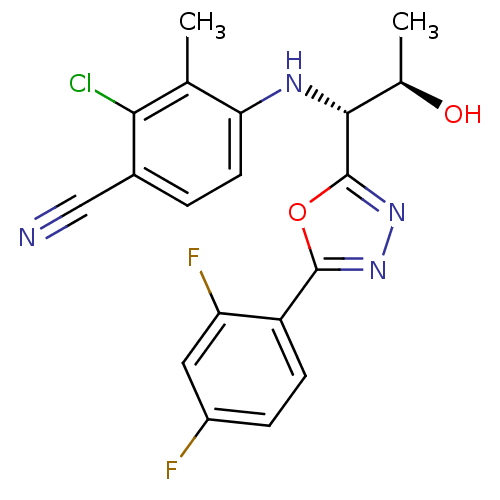
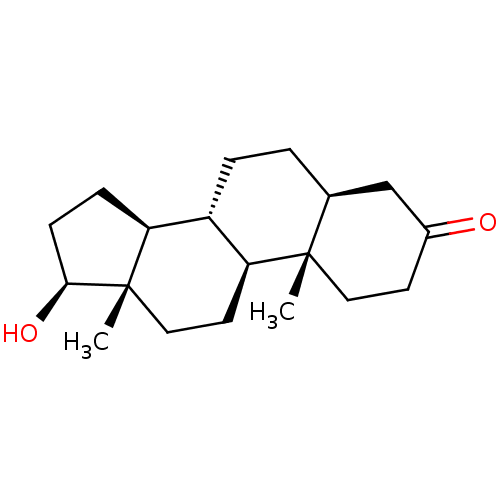
 3D Structure (crystal)
3D Structure (crystal)