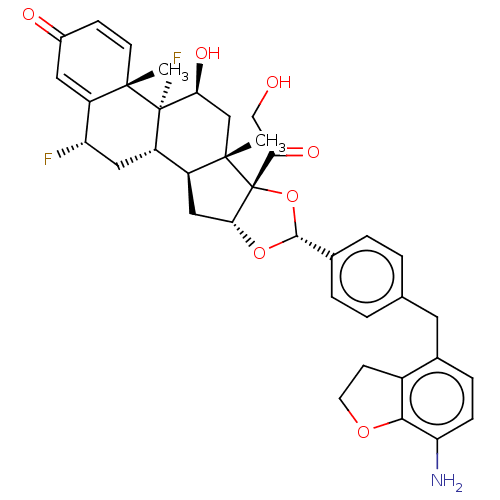
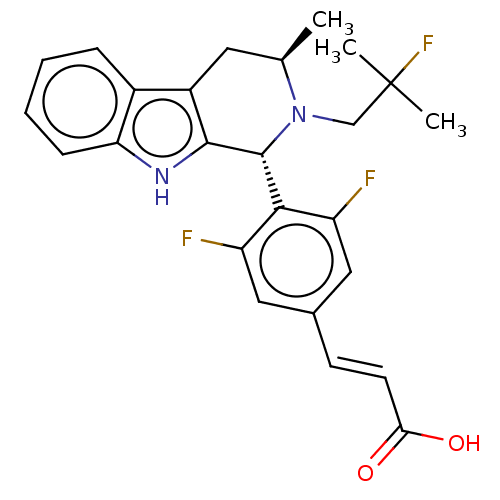
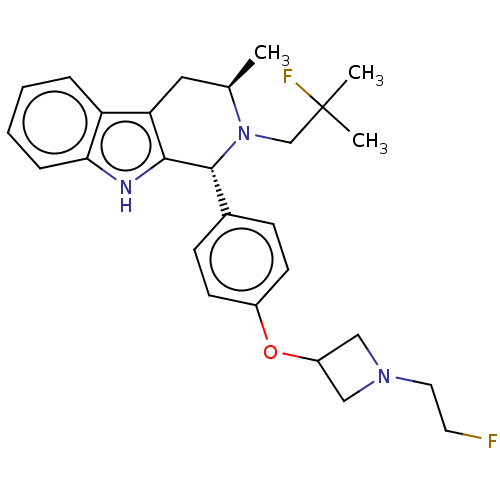
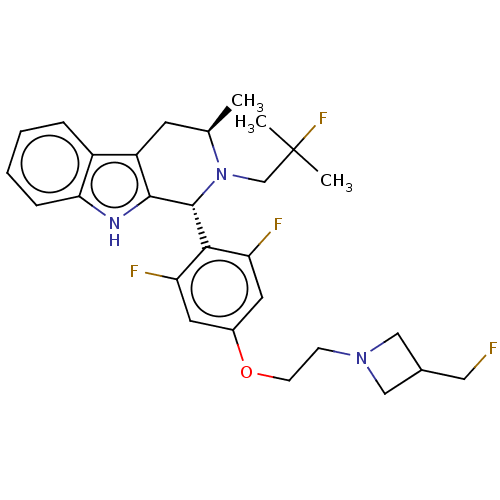
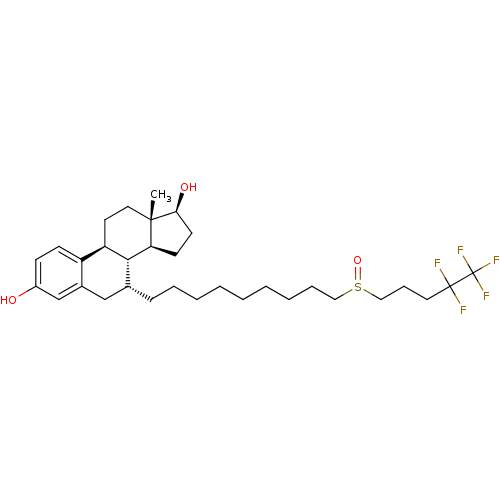
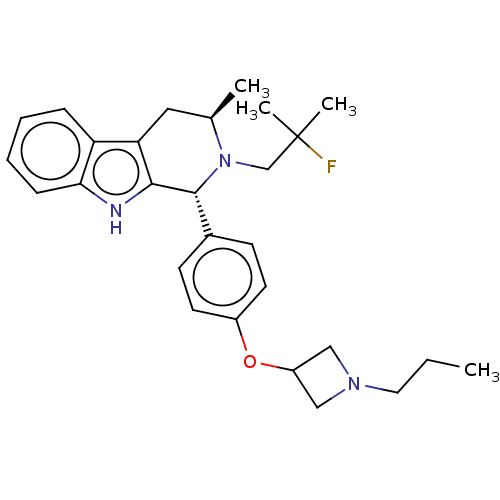
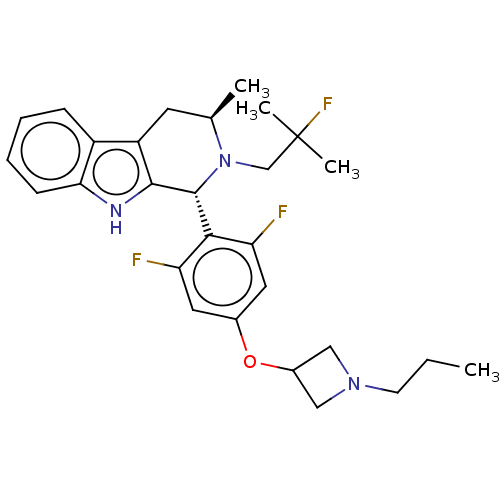
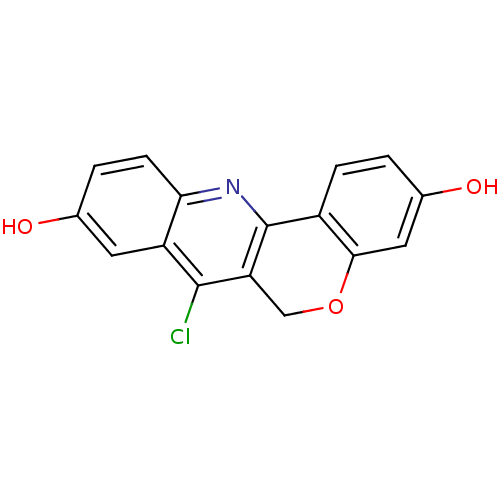
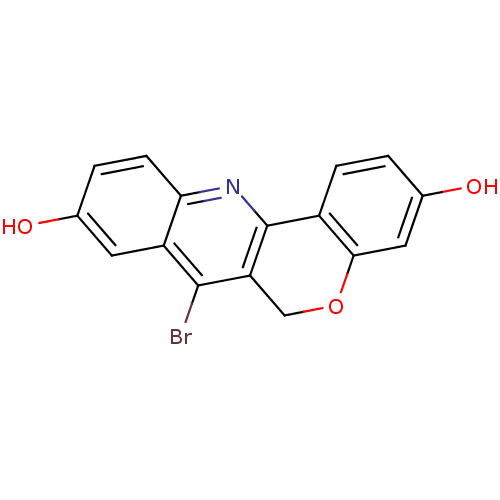
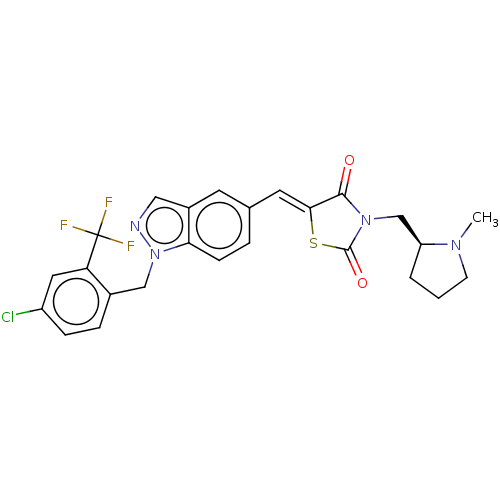
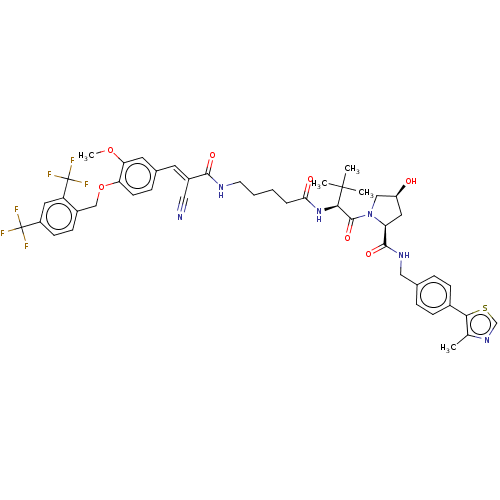
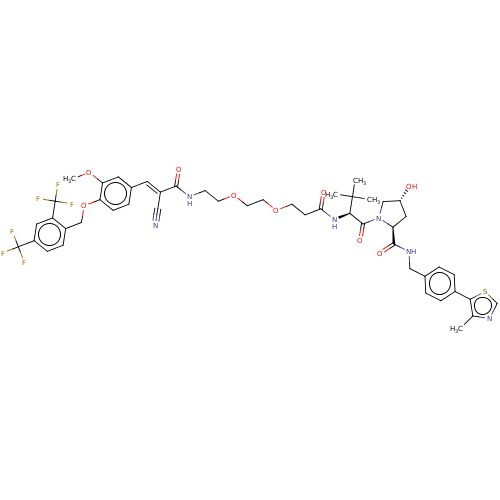
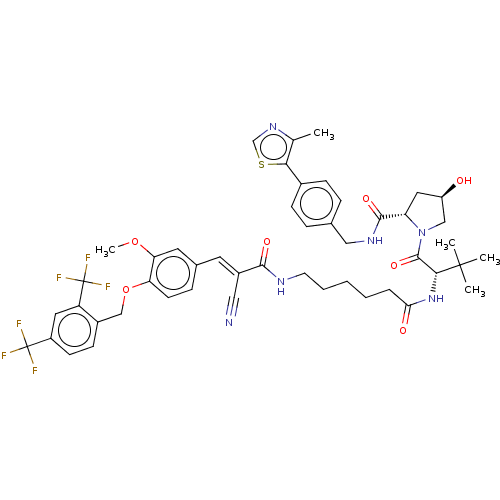
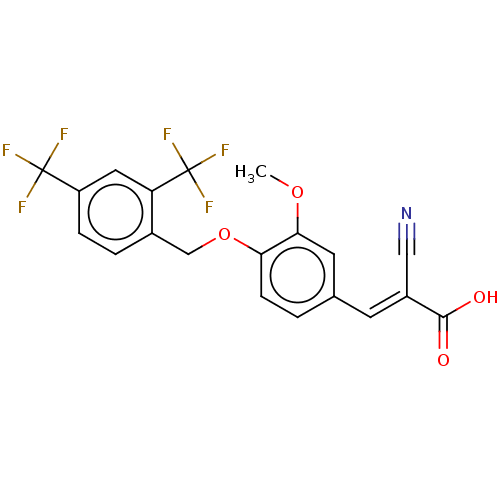
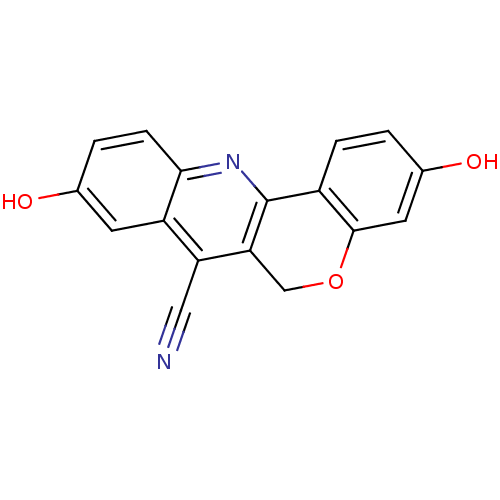
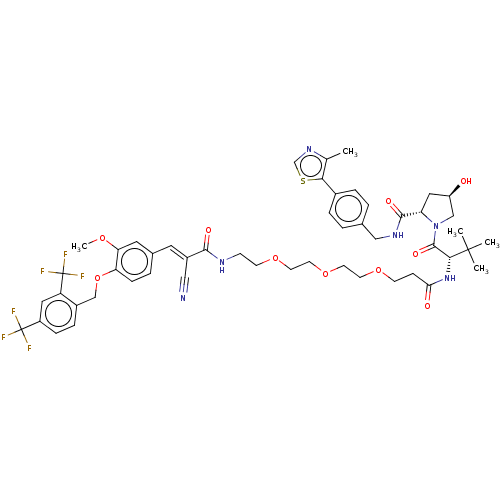
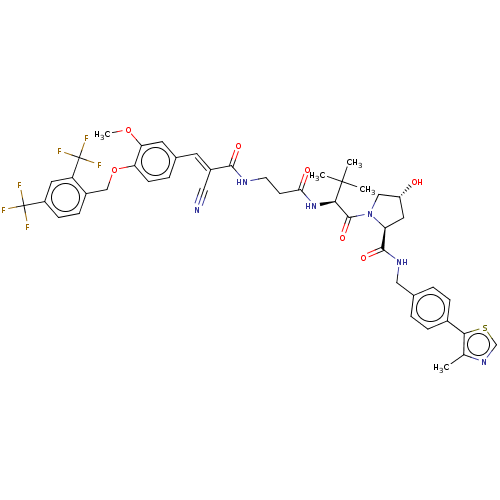
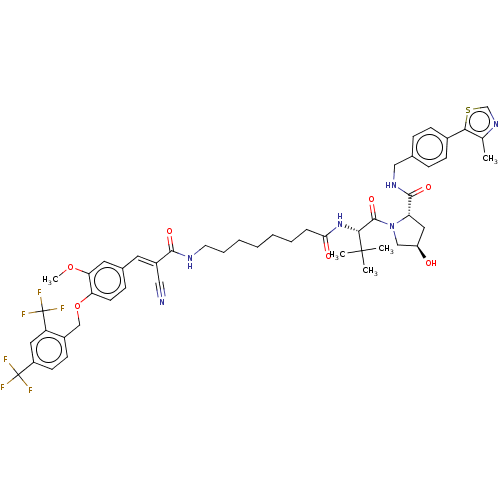
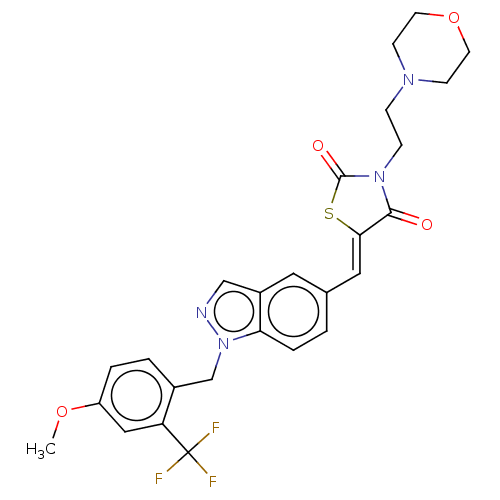
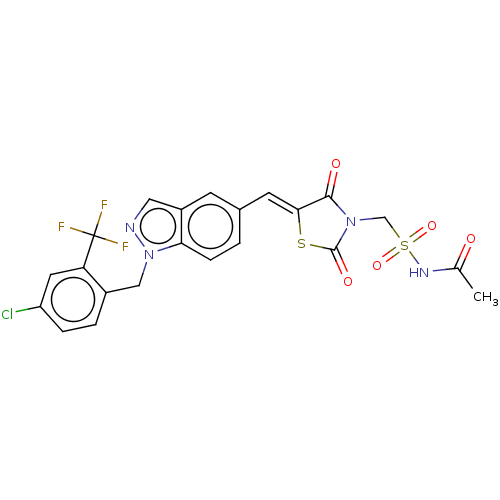
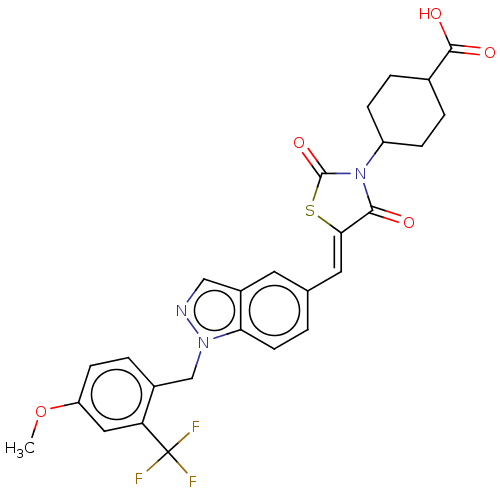
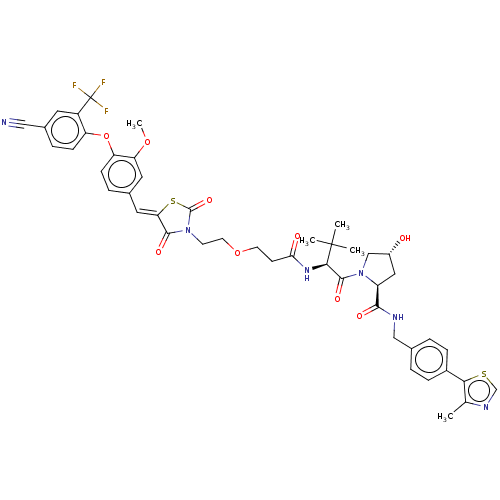
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 900nMAssay Description:Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assayMore data for this Ligand-Target Pair
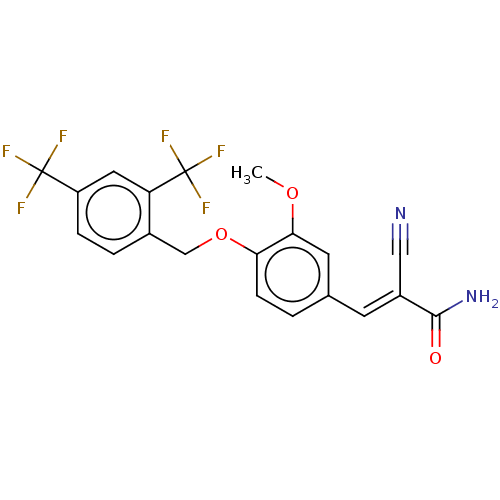
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assayMore data for this Ligand-Target Pair
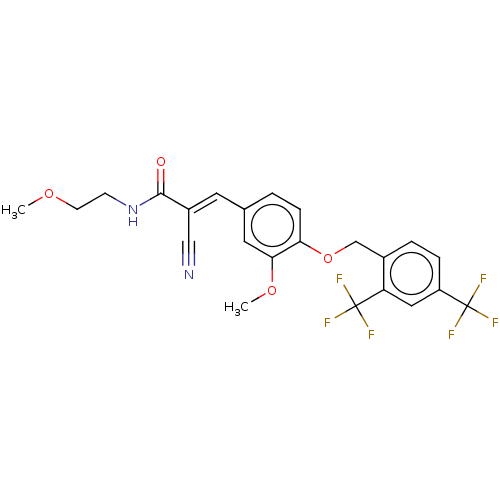
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 5.90nMAssay Description:Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assayMore data for this Ligand-Target Pair
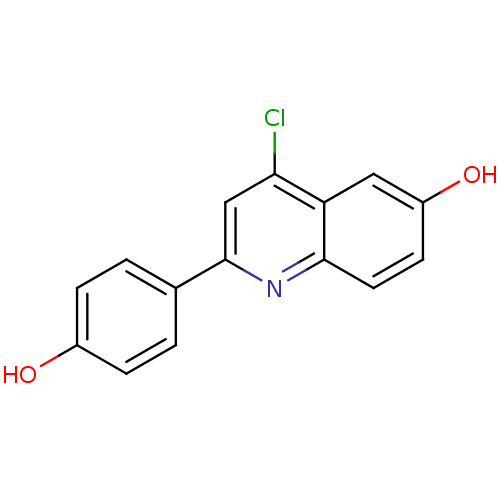
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 5.20E+3nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 1.10E+3nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 5.90E+3nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 5.40E+3nMAssay Description:Agonist activity at recombinant full-length human ERRalpha expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 191nMAssay Description:The binding activity of the compound to ER was determined using the LanthaScreen TR-FRET ER Alpha Coactivator Assay kit (brand: Thermo, Cat. No: A158...More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 490nMAssay Description:The binding activity of the compound to ER was determined using the LanthaScreen TR-FRET ER Alpha Coactivator Assay kit (brand: Thermo, Cat. No: A158...More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 111nMAssay Description:The binding activity of the compound to ER was determined using the LanthaScreen TR-FRET ER Alpha Coactivator Assay kit (brand: Thermo, Cat. No: A158...More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 427nMAssay Description:The binding activity of the compound to ER was determined using the LanthaScreen TR-FRET ER Alpha Coactivator Assay kit (brand: Thermo, Cat. No: A158...More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 52nMAssay Description:The binding activity of the compound to ER was determined using the LanthaScreen TR-FRET ER Alpha Coactivator Assay kit (brand: Thermo, Cat. No: A158...More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.372nMAssay Description:Compounds were screened for their ability to displace a fluorescent labelled tracer ERα ligand via time resolved fluorescent energy transfer usi...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.35nMAssay Description:Compounds were screened for their ability to displace a fluorescent labelled tracer ERα ligand via time resolved fluorescent energy transfer usi...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.62nMAssay Description:Compounds were screened for their ability to displace a fluorescent labelled tracer ERα ligand via time resolved fluorescent energy transfer usi...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at ERalpha receptor in human MCF7 cells assessed as inhibition of cell growth after 6 days by crystal violet staining methodMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.29nMAssay Description:Compounds were screened for their ability to displace a fluorescent labelled tracer ERα ligand via time resolved fluorescent energy transfer usi...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.69nMAssay Description:Compounds were screened for their ability to displace a fluorescent labelled tracer ERα ligand via time resolved fluorescent energy transfer usi...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.82nMAssay Description:Compounds were screened for their ability to displace a fluorescent labelled tracer ERα ligand via time resolved fluorescent energy transfer usi...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human ERalphaMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human ERalphaMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human ERalphaMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Inhibition of human ERalphaMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human ERalphaMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of [3H]E2 from human recombinant ERalpha receptor after overnight incubation by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Inhibition of human ERalphaMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 7.70nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at His6-tagged ERRalpha LBD (unknown origin) assessed as inhibition of GST-tagged SRC-2 co-activator peptide recruitment after 18...More data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Binding affinity to GST-tagged ERRalpha-LBD (unknown origin) using fluorescein-conjugated coactivator PGC-1alpha incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
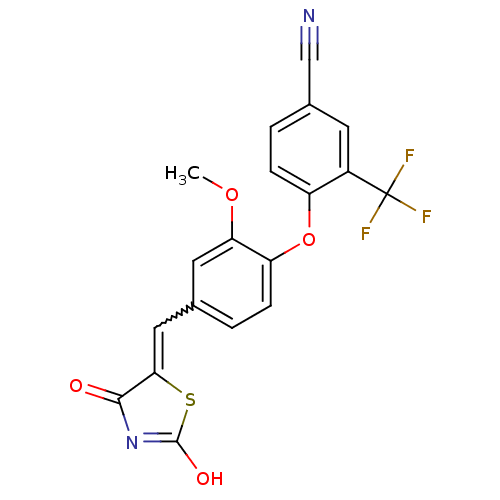
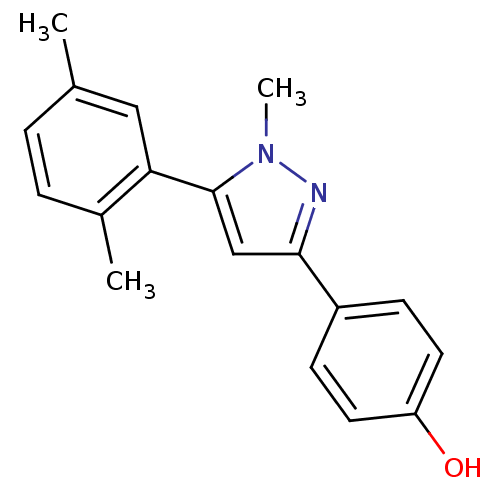

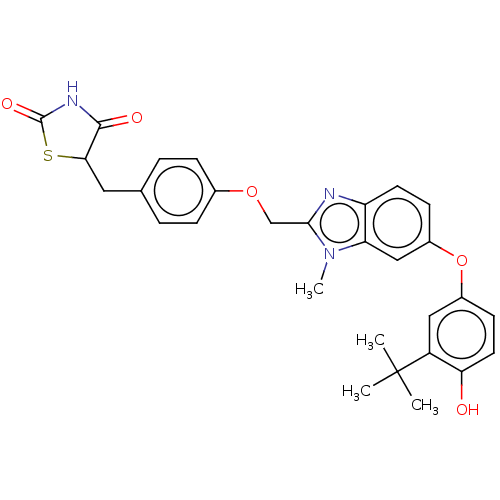
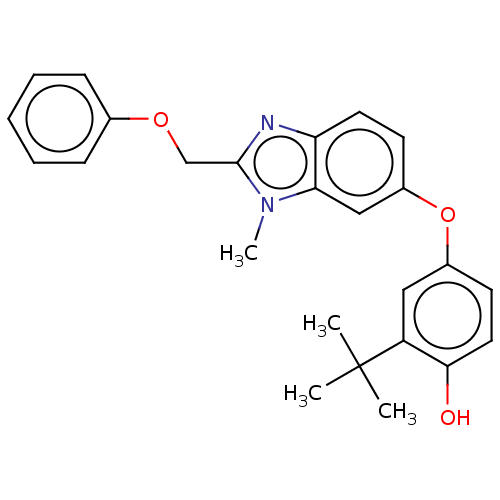
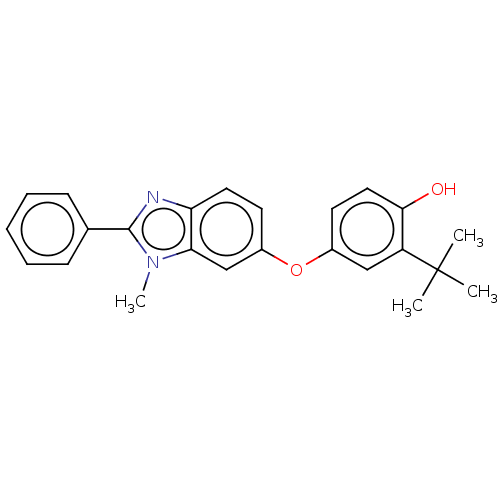
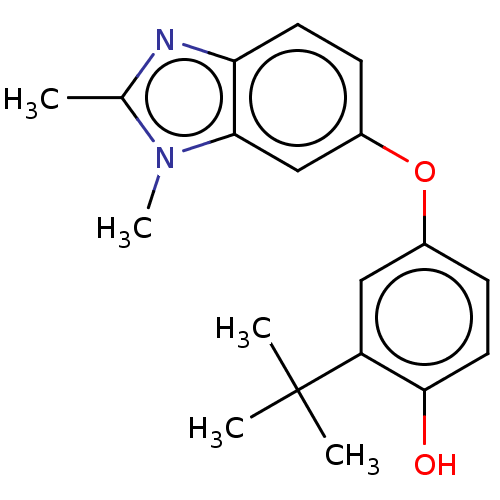
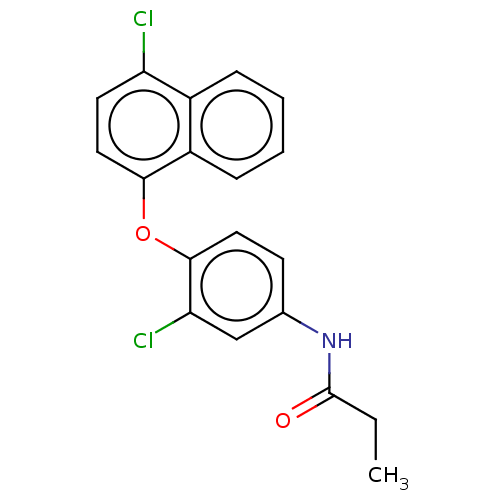
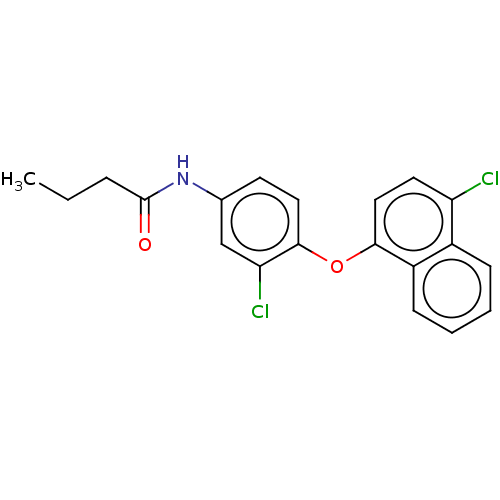
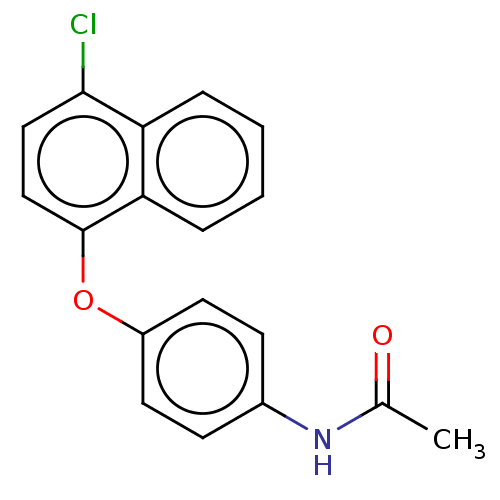
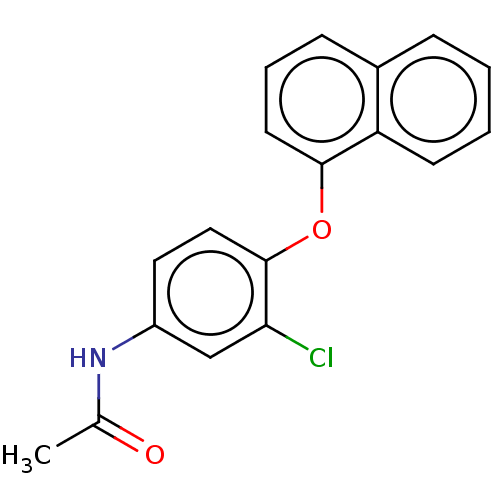
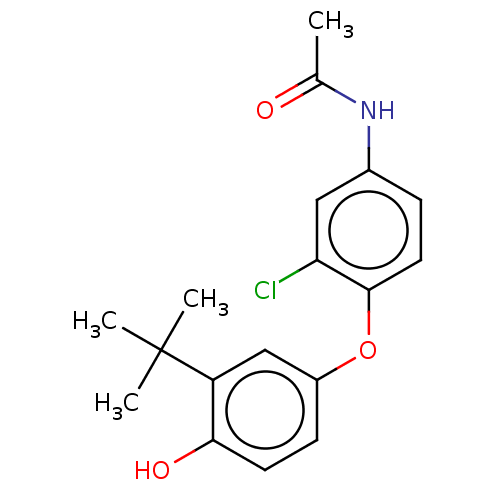
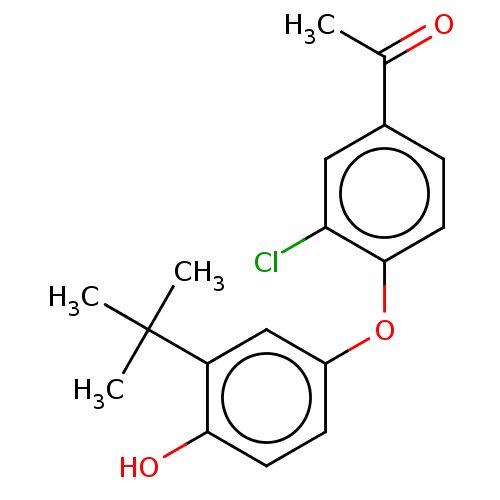
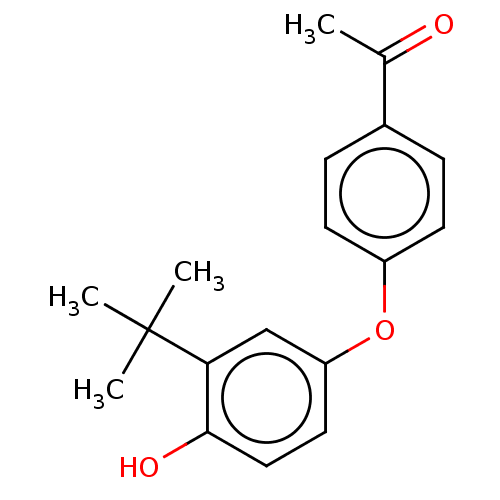
 3D Structure (crystal)
3D Structure (crystal)